Assessment of Potentially Toxic Elements in the Urban Soil and Plants of Kirkuk City in Iraq
Abstract
1. Introduction
2. Materials and Methods
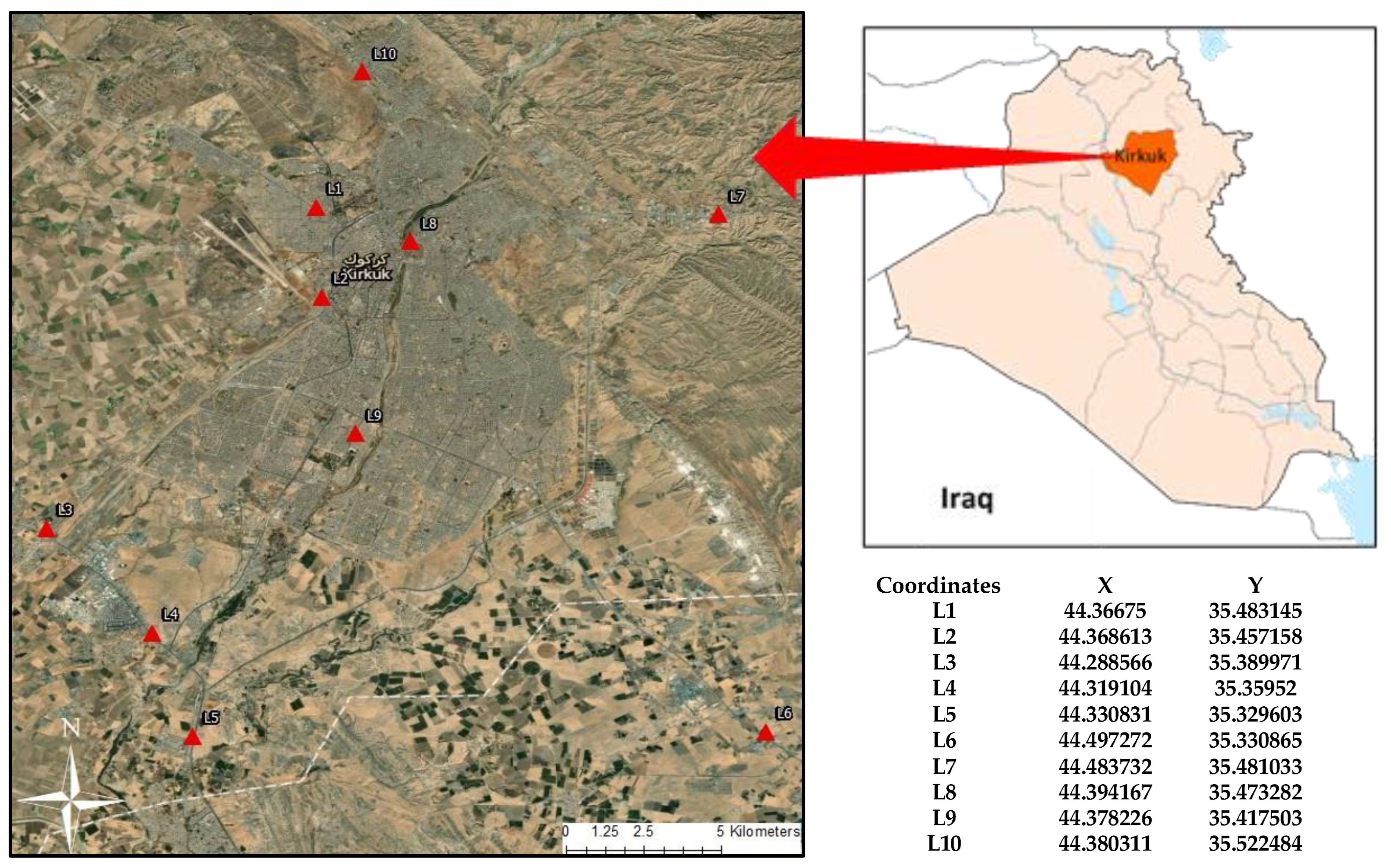
2.1. Sample Sites
2.2. Soil Physico-Chemical Characterization
2.3. Acid Digestion of All Samples
2.4. ICP-MS Analysis
2.5. Measuring Pollution Indices
2.6. Statistical Analysis
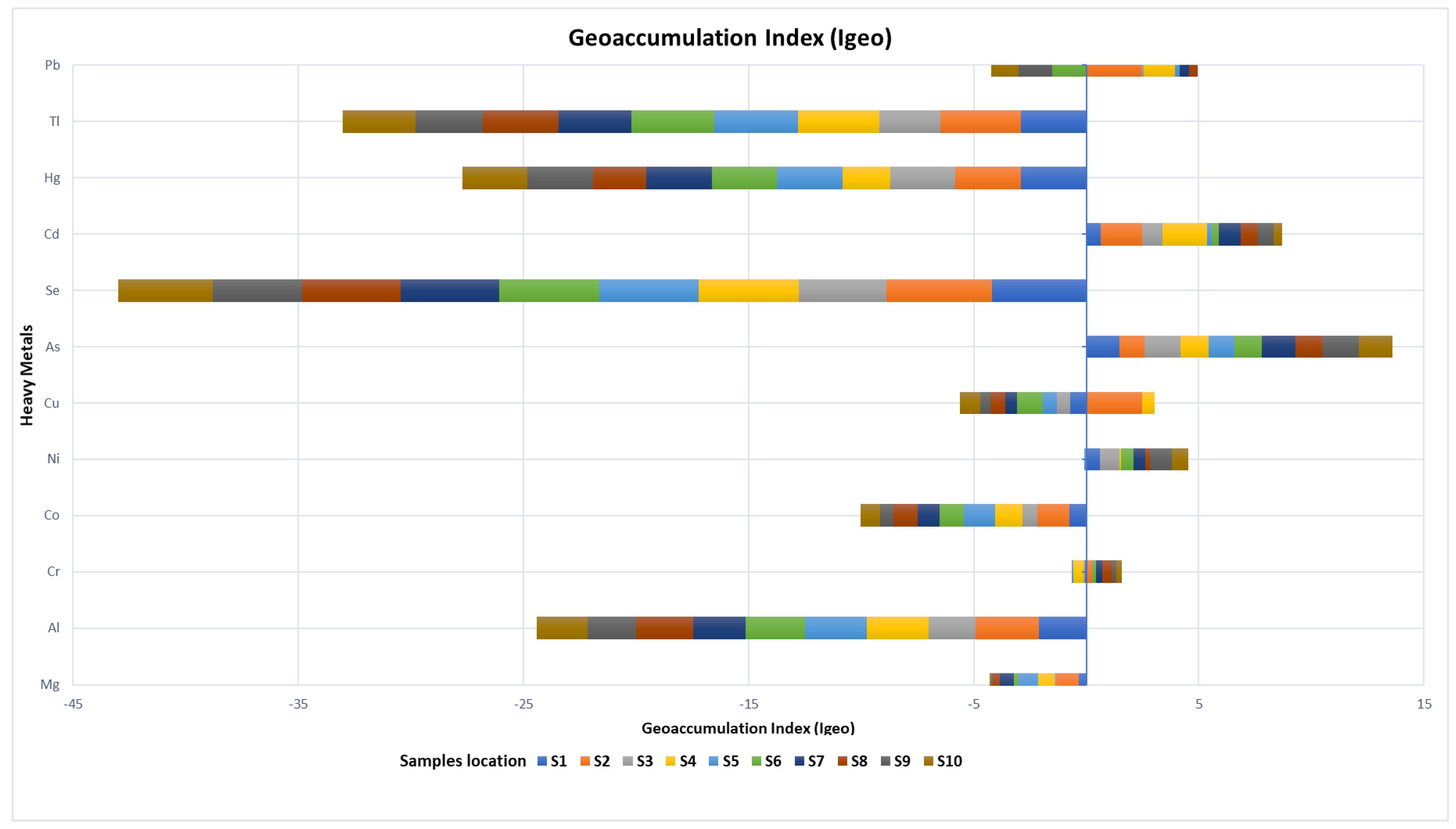
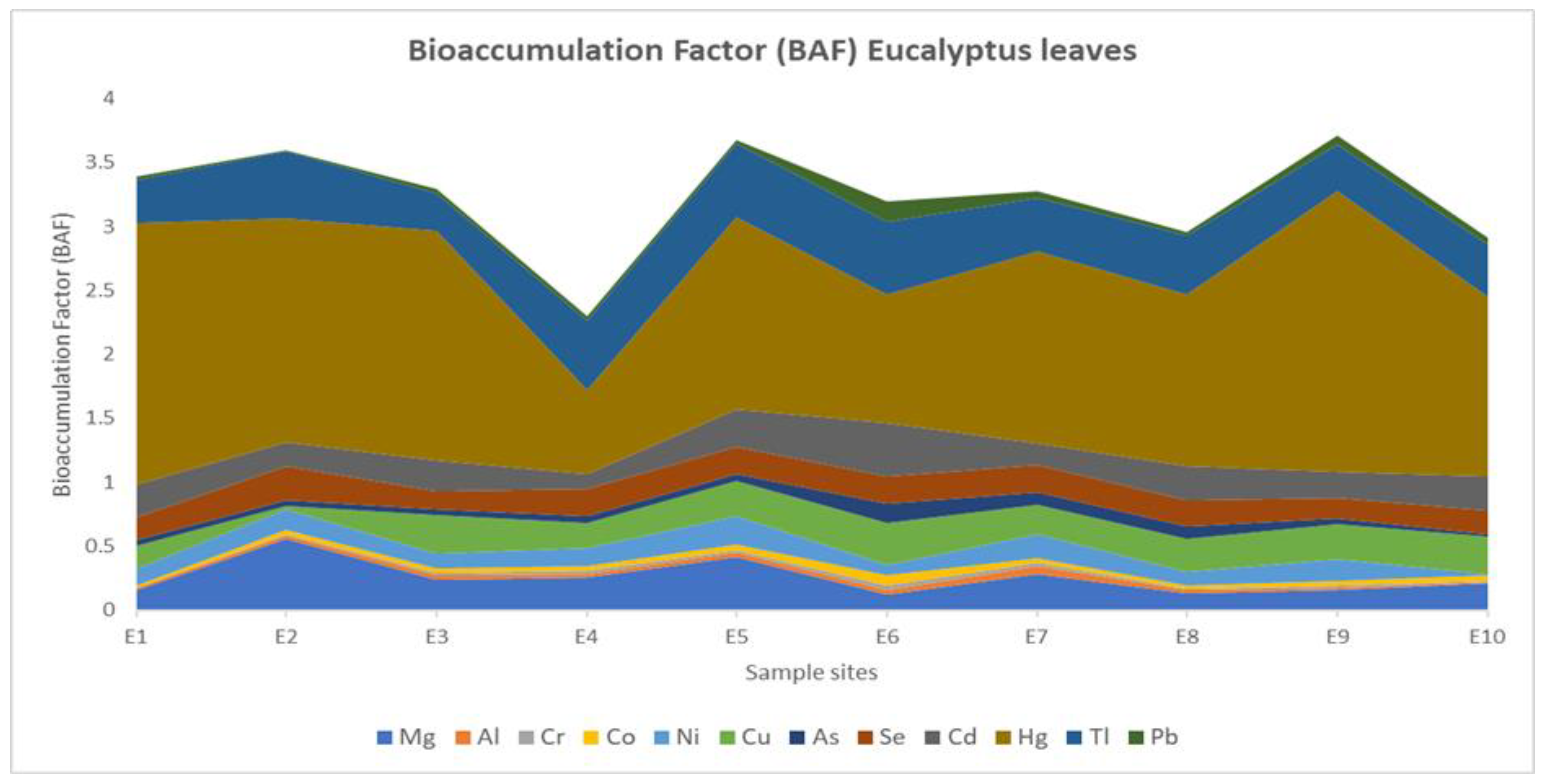
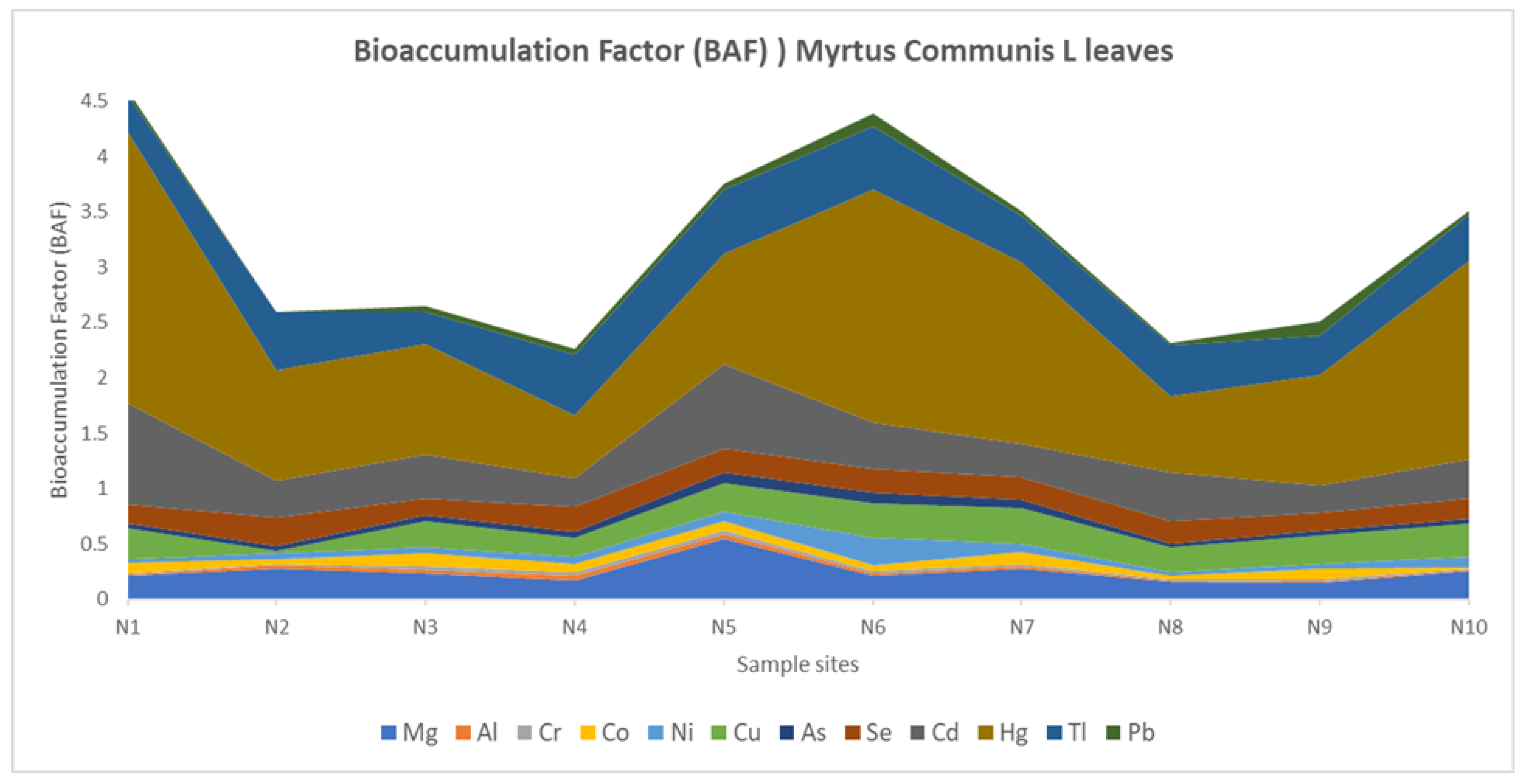
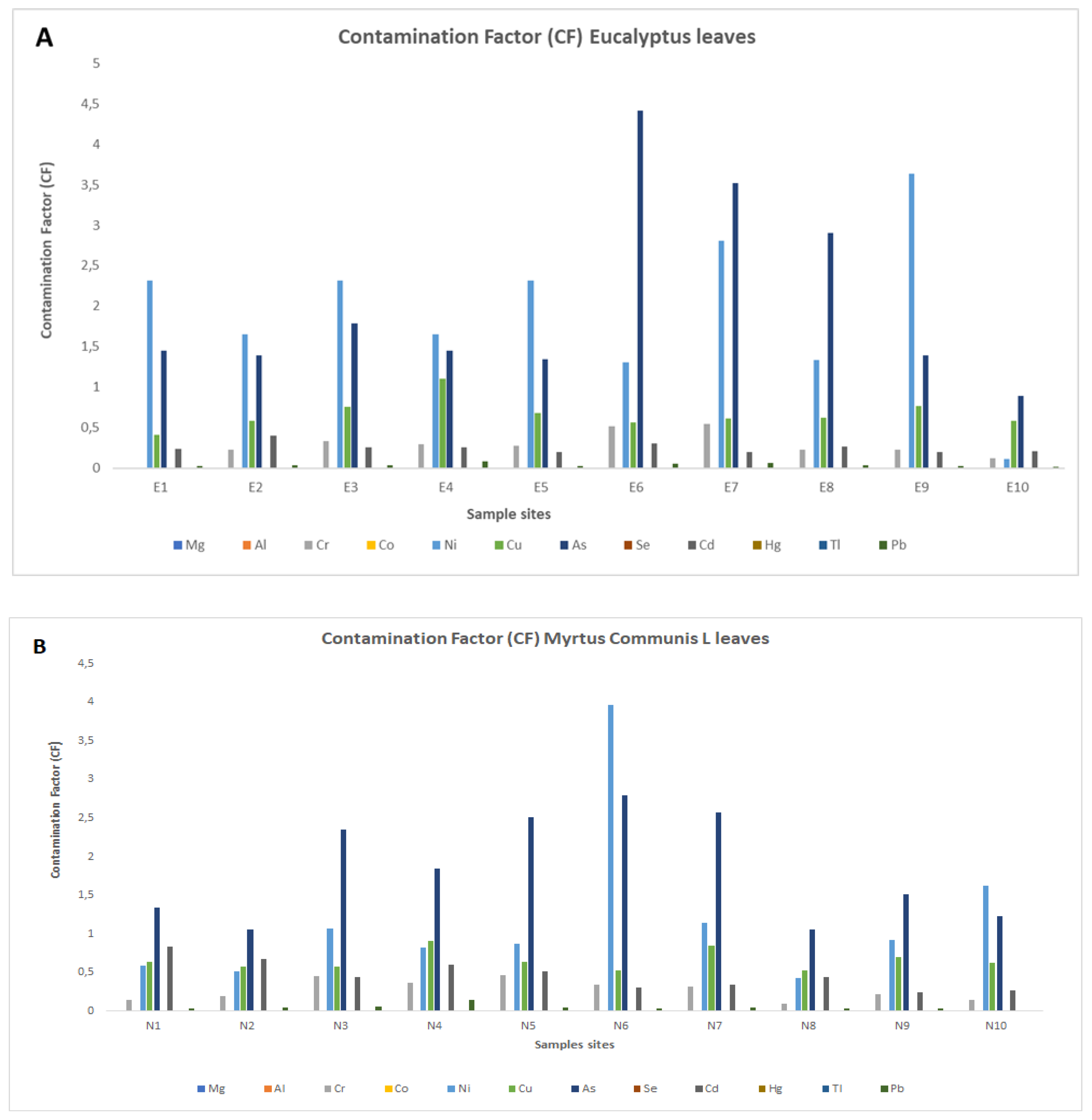
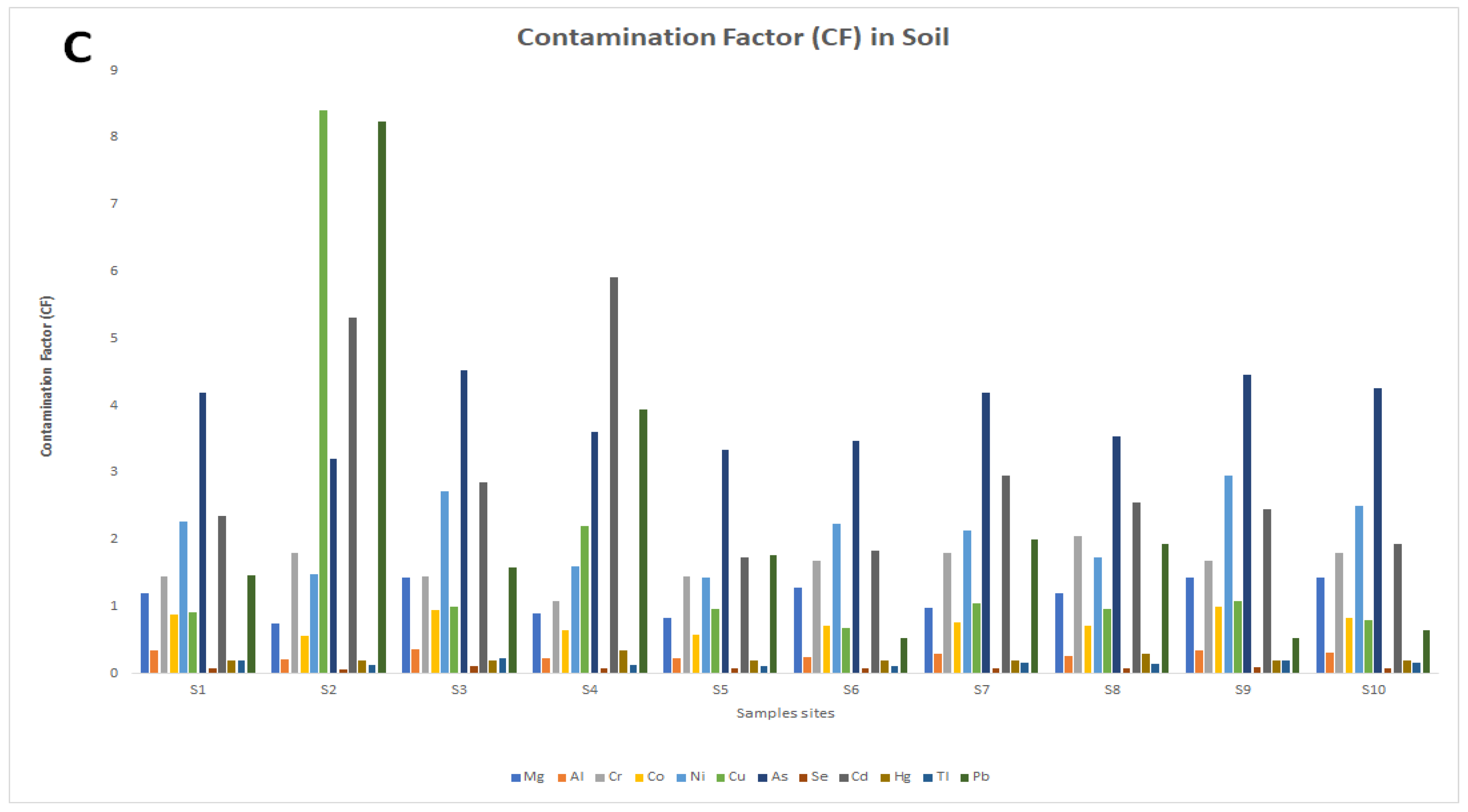
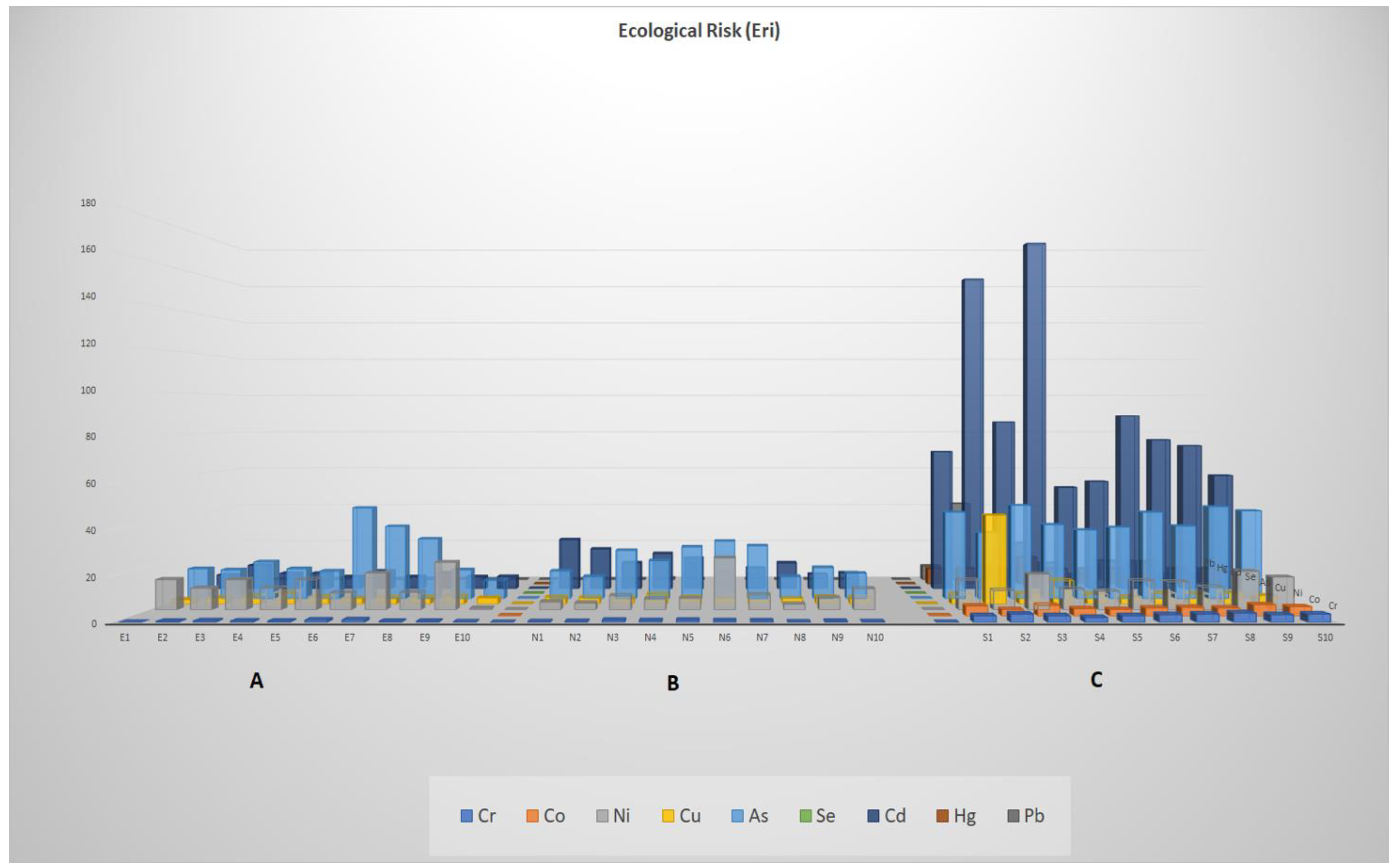
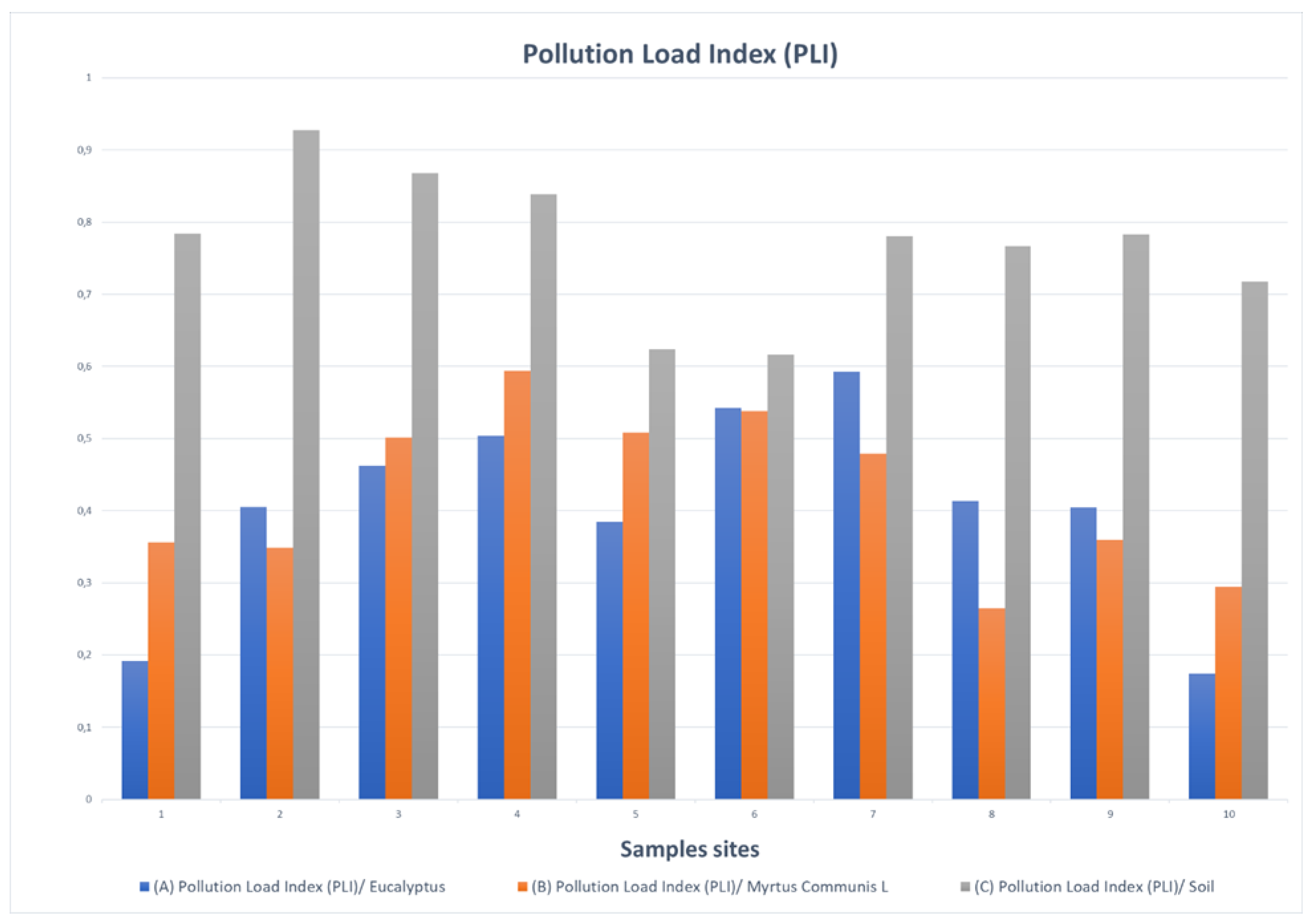
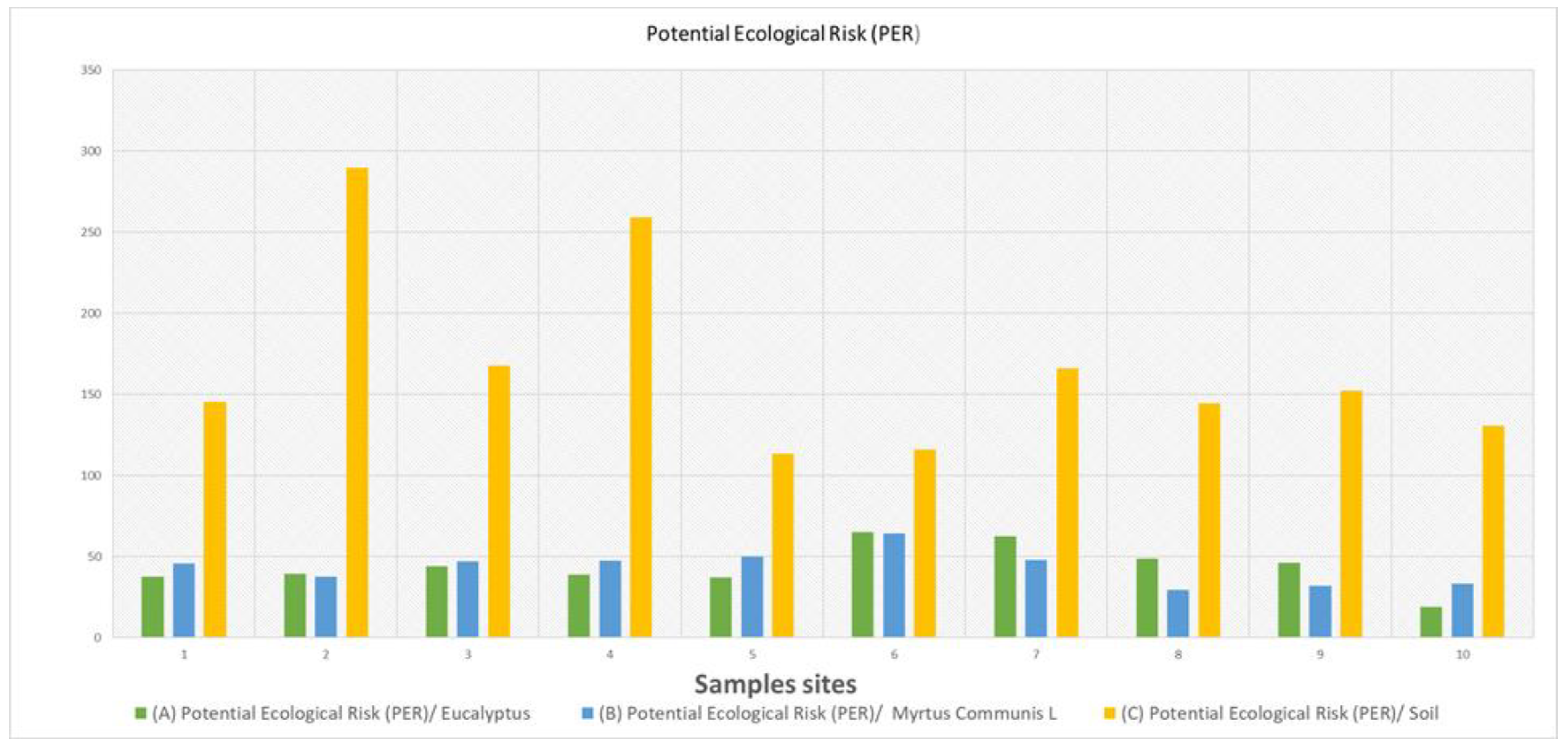
3. Results
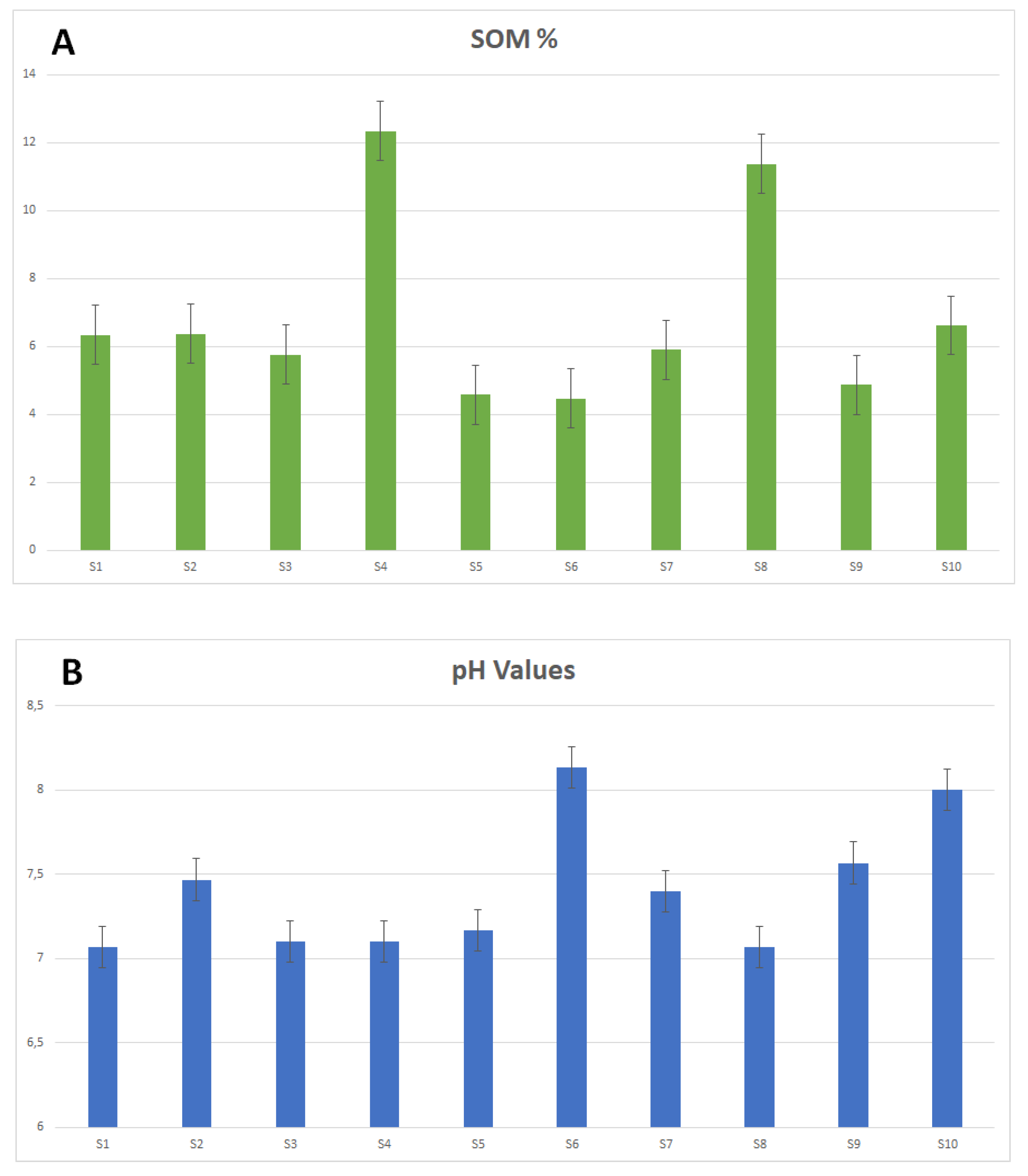
3.1. Physico-Chemical Soil Characterization
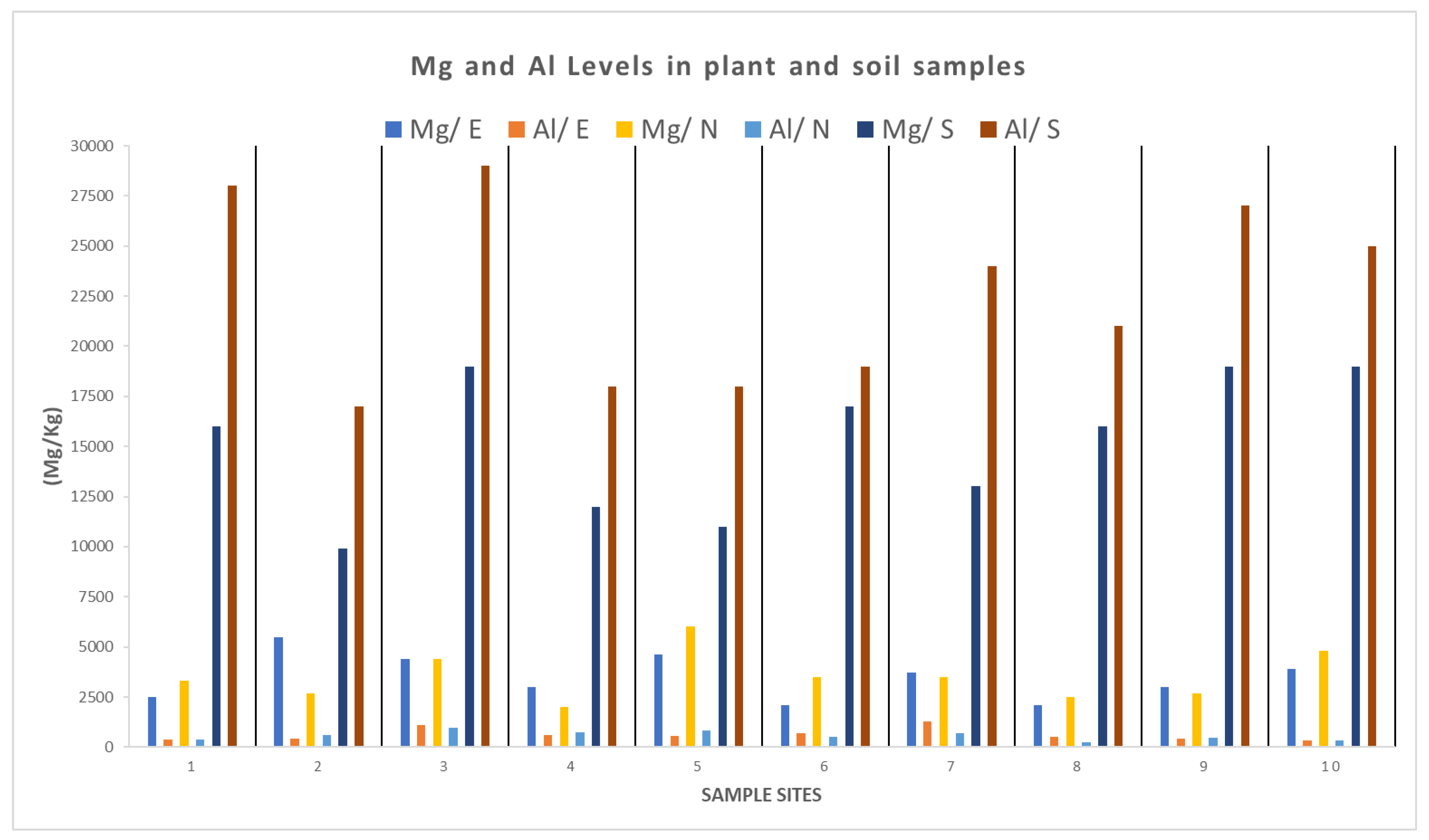
3.2. Potentially Toxic Element (PTE) Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, A.H.; Khalid, R.S.; Alaama, M.; Abdualkader, A.M.; Kasmuri, A.; Abbas, S.A. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 2016, 7, 1. [Google Scholar] [CrossRef]
- Badamasi, H. Biomonitoring of Air pollution using plants. J. Environ. Sci. 2017, 2, 27–39. [Google Scholar]
- Wong, M.H.; Leung, A.O.; Wu, S.; Leung, C.K.; Naidu, R. Mitigating environmental and health risks associated with uncontrolled recycling of electronic waste: Are international and national regulations effective. In Environmental Contamination: Health Risks and Ecological Restoration; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 193–208. [Google Scholar]
- Barbes, L.; Barbulescu, A.; Radulescu, C.; Stihi, C.; Chelarescu, E.D. Determination of heavy metals in leaves and bark of Populus nigra L. by atomic absorption spectrometry. Rom. Rep. Phys. 2014, 66, 877–886. [Google Scholar]
- Kabir, M.H.; Kormoker, T.; Shammi, R.S.; Tusher, T.R.; Islam, M.S.; Khan, R.; Idris, A.M. A comprehensive assessment of heavy metal contamination in road dusts along a hectic national highway of Bangladesh: Spatial distribution, sources of contamination, ecological and human health risks. Toxin Rev. 2021, 40, 1–20. [Google Scholar] [CrossRef]
- Hseu, Z.-Y. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol. 2004, 95, 53–59. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Amantea, D.; Caruso, A.; Saturnino, C. Chemical and biological properties of toxic metals and use of chelating agents for the pharmacological treatment of metal poisoning. Arch. Toxicol. 2010, 84, 501–520. [Google Scholar] [CrossRef]
- Knudson, L.L.; Tibbitts, T.W.; Edwards, G.E. Measurement of Ozone Injury by Determination of Leaf Chlorophyll Concentration. Plant Physiol. 1977, 60, 606–608. [Google Scholar] [CrossRef]
- Denneman, C.A.; Robberse, J.G. Ecotoxicological risk assessment as a base for development of soil quality criteria. In Contaminated Soil’90; Springer: Dordrecht, The Netherlands, 1990; pp. 157–164. [Google Scholar]
- Van Beek, C.L.; Elias, E.; Yihenew, G.S.; Heesmans, H.; Tsegaye, A.; Feyisa, H.; Mengist, S. Soil nutrient balances under diverse agro-ecological settings in Ethiopia. Nutr. Cycl. Agroecosyst. 2016, 106, 257–274. [Google Scholar] [CrossRef]
- Andrews, S.S.; Carroll, C.R. Designing a soil quality assessment for sustainable agroecosystem management. Ecol. Appl. 2002, 11, 1573–1585. [Google Scholar] [CrossRef]
- Teng, Y.; Wu, J.; Lu, S.; Wang, Y.; Jiao, X.; Song, L. Soil and soil environmental quality monitoring in China: A review. Environ. Int. 2014, 69, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yin, C.; Chen, M.; Wang, W. Risk assessment of heavy metals in street dust particles to a stream network. Soil Sediment Contam. 2009, 18, 173–183. [Google Scholar] [CrossRef]
- Morvan, X.; Saby, N.P.A.; Arrouays, D.; Le Bas, C.; Jones, R.J.A.; Verheijen, F.G.A.; Bellamy, P.H.; Stephens, M.; Kibblewhite, M.G. Soil monitoring in Europe: A review of existing systems and requirements for harmonization. Sci. Total Environ. 2008, 391, 1–12. [Google Scholar] [CrossRef] [PubMed]
- van der Fels-Klerx, H.; Meijer, N.; Nijkamp, M.; Schmitt, E.; van Loon, J. Chemical food safety of using former foodstuffs for rearing black soldier fly larvae (Hermetia illucens) for feed and food use. J. Insects Food Feed 2020, 6, 475–488. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Atiemo, M.S.; Ofosu, G.F.; Kuranchie-Mensah, H.; Tutu, A.O.; Palm, N.D.; Blankson, S.A. Contamination assessment of heavy metals in road dust from selected roads in Accra, Ghana. Res. J. Environ. Earth Sci. 2011, 3, 473–480. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Malden, MA, USA, 1985. [Google Scholar]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosystems 2001, 2, 108–118. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Ghoochian, M.; Taghavi, L. Assessment of Heavy Metal Contamination in Surface Sediment of the Darreh-Morad Beyg River. Iran. J. Heal. Sci. 2016, 4, 22–34. [Google Scholar] [CrossRef][Green Version]
- Sultan, M.B.; Choudhury, T.R.; Alam, N.-E.; Doza, B.; Rahmana, M. Soil, dust, and leaf-based novel multi-sample approach for urban heavy metal contamination appraisals in a megacity, Dhaka, Bangladesh. Environ. Adv. 2021, 7, 100154. [Google Scholar] [CrossRef]
- Håkanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Afloog, H.A.; Hassan, A.A. Environmental Geochemistry and Assessment of Pollution by Vanadium in Top Soil of Kirkuk, Northern Iraq. Iraqi Geol. J. 2020, 53, 74–95. [Google Scholar] [CrossRef]
- Núñez, A.; Cotrufo, M.F.; Schipanski, M. Irrigation effects on the formation of soil organic matter from aboveground plant litter inputs in semiarid agricultural systems. Geoderma 2022, 416, 115804. [Google Scholar] [CrossRef]
- Raheem, A.M.; Omar, N.Q. Investigation of distinctive physico-chemical soil correlations for Kirkuk city using spatial analysis technique incorporated with statistical modeling. Int. J. Geo-Engineering 2021, 12, 1–21. [Google Scholar] [CrossRef]
- Wosten, J.H.M.; Groenendijk, P.; Veraart, J.A.; van der Lugt, L.M. Soil Organic Matter and its Importance For Water Management; Stowa: Engelsbrand, Germany, 2019. [Google Scholar]
- Oades, J.M. An Introduction to Organic Matter in Mineral Soils. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1989; Volume 1, pp. 89–159. [Google Scholar]
- Li, G.; Lu, N.; Wei, Y.; Zhu, D. Relationship between heavy metal content in polluted soil and soil organic matter and pH in mining areas. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 052081. [Google Scholar] [CrossRef]
- Skorbiłowicz, M.; Skorbiłowicz, E. Content of calcium, magnesium, sodium and potassium in the street dust from the area of Białystok (Poland). J. Ecol. Eng. 2019, 20, 125–131. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley: New York, NY, USA, 1979. [Google Scholar]
- Rout, G.R.; Samantaray, S.; Das, P. Aluminium toxicity in plants: A review. Agronomie 2001, 21, 3–21. [Google Scholar] [CrossRef]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of High and Low Levels of Plant-Beneficial Heavy Metal Ions on Plant Growth and Development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Grembecka, M.; Szefer, P. Monitoring of essential and heavy metals in green tea from different geographical origins. Environ. Monit. Assess. 2016, 188, 183. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Permissible Limits of Heavy Metals in Soil and Plants; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Cheng, J.-L.; Shi, Z.; Zhu, Y.-W. Assessment and mapping of environmental quality in agricultural soils of Zhejiang Province, China. J. Environ. Sci. 2007, 19, 50–54. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Ahmad, A.; Alyemeni, M.N. Soil cadmium enrichment: Allocation and plant physiological manifestations. Saudi J. Biol. Sci. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Awadh, S.M.; Al-Hamdani, J.A.J.M.Z. Urban geochemistry assessment using pollution indices: A case study of urban soil in Kirkuk, Iraq. Environ. Earth Sci. 2019, 78, 1–12. [Google Scholar] [CrossRef]
- Kaur, J.; Bhat, S.A.; Singh, N.; Bhatti, S.S.; Kaur, V.; Katnoria, J.K. Assessment of the Heavy Metal Contamination of Roadside Soils Alongside Buddha Nullah, Ludhiana, (Punjab) India. Int. J. Environ. Res. Public Heal. 2022, 19, 1596. [Google Scholar] [CrossRef] [PubMed]
- Al-Obeidi, A.H.; Al-Jumaily, H.A. Geochemistry and environmental assessment of heavy metals in surface soil in Al-Hawija, southwest Kirkuk. Iraqi Geol. J. 2020, 50, 36–61. [Google Scholar] [CrossRef]
- Wilson, S.C.; Tighe, M.; Paterson, E.; Ashley, P.M. Food crop accumulation and bioavailability assessment for antimony (Sb) compared with arsenic (As) in contaminated soils. Environ. Sci. Pollut. Res. 2014, 21, 11671–11681. [Google Scholar] [CrossRef] [PubMed]
- Hartley, T.N.; Macdonald, A.; McGrath, S.; Zhao, F.-J. Historical arsenic contamination of soil due to long-term phosphate fertiliser applications. Environ. Pollut. 2013, 180, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, J.; Mishra, P.P. Toxic metals in crops: A burgeoning problem. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Springer: Singapore, 2020; pp. 273–301. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. “Introduction to Phytoremediation,” National Risk Management Research Laboratory, EPA/600/R-99/107. 2000. Available online: https://www.clu-in.org/download/remed/introphyto.pdf (accessed on 30 March 2022).
- Luo, J.; Qi, S.; Peng, L.; Xie, X. Enhanced phytoremediation capacity of a mixed-species plantation of Eucalyptus globulus and Chickpeas. J. Geochem. Explor. 2017, 182, 201–205. [Google Scholar] [CrossRef]
- Hasan, M.A.; Mahal, J.D.; Ali, H.A. Phytoremediation of Pb and Ni heavy metals found in soil using Myrtus communis. Eur. Asian J. BioSciences 2020, 14, 2069–2073. [Google Scholar]
- U.S. Department of Health and Human Services. Public Health Service Agency for Toxic Substances and Disease Registry. Division of Toxicology and Environmental Medicine. Arsenic. 2005. Available online: https://www.baltimorehealth.org/info/ATSDR%20fact%20sheet.pdf (accessed on 30 March 2022).
- Andrianisa, H.A.; Ito, A.; Sasaki, A.; Aizawa, J.; Umita, T. Biotransformation of arsenic species by activated sludge and removal of bio-oxidised arsenate from wastewater by coagulation with ferric chloride. Water Res. 2008, 42, 4809–4817. [Google Scholar] [CrossRef]
- National Ground Water Association, Copyright 2001. Arsenic. What You Need to Know. Available online: http://www.ngwa.org/ASSETS/A0DD107452D74B33AE9D5114EE6647ED/Arsenic.pdf (accessed on 28 February 2022).
- Pehlivan, E.; Özkan, A.M.; Dinç, S.; Parlayici, Ş. Adsorption of Cu2+ and Pb2+ ion on dolomite powder. J. Hazard. Mater. 2009, 167, 1044–1049. [Google Scholar] [CrossRef]
- Traunfeld, J.H.; Clement, D.L. “Lead in Garden Soils. Home and Garden,” Maryland Cooperative Extention. University of Maryland, 2001. Available online: https://www.hgic.umd.edu/media/documents/hg18.pdf (accessed on 28 February 2022).
- Salido, A.L.; Hasty, K.L.; Lim, J.-M.; Butcher, D.J. Phytoremediation of Arsenic and Lead in Contaminated Soil Using Chinese Brake Ferns (Pteris vittata) and Indian Mustard (Brassica juncea). Int. J. Phytoremediation 2003, 5, 89–103. [Google Scholar] [CrossRef]
- Interstate Technology and Regulatory Council. Phytotechnology Technical and Regulatory Guidance and Decision Trees. 2009. Available online: https://www.itrcweb.org/guidancedocument.asp?TID=63 (accessed on 28 March 2022).
| Sample Code/Soil | Sample Code/Eucalyptus Trees | Sample Code/Myrtus communis L. Trees | Sample Sites | Contributing Sources of Pollution |
|---|---|---|---|---|
| S1 | E1 | N1 | North Oil Company | Industrial and domestic |
| S2 | E2 | N2 | Bus and long vehicle terminals | Roadside |
| S3 | E3 | N3 | Southwest city motorway | Roadside |
| S4 | E4 | N4 | Southern industrial site | Industrial and roadside |
| S5 | E5 | N5 | Electricity power station | Industrial |
| S6 | E6 | N6 | Cement production company | Industrial |
| S7 | E7 | N7 | Northeast city motorway | Roadside and industrial |
| S8 | E8 | N8 | Old town center | Roadside and domestic |
| S9 | E9 | N9 | New city center | Roadside and domestic |
| S10 | E10 | N10 | Northwest city motorway | Roadside and industrial |
| (A) Element Concentration (mg/kg) in Eucalyptus Leaves | ||||||||||||
| Elements | Mg ± Std | Al ± Std | Cr ± Std | Co ± Std | Ni ± Std | Cu ± Std | As ± Std | Se ± Std | Cd ± Std | Hg ± Std | Tl ± Std | Pb ± Std |
| E1 | 2500 ± 56.6 | 380 ± 1.3 | 0.05 ± 0.00 | 0.26 ± 0.01 | 14 ± 0.5 | 4.1 ± 0.1 | 0.26 ± 0.01 | 0.75 ± 0.011 | 0.058 ± 0.002 | 0.041 ± 0.001 | 0.05 ± 0.003 | 0.72 ± 0.025 |
| E2 | 5500 ± 121.6 | 410 ± 1.4 | 1.9 ± 0.01 | 0.35 ± 0.01 | 10 ± 0.4 | 5.8 ± 0.2 | 0.20.01 | 0.75 ± 0.042 | 0.10 ± 0.004 | 0.035 ± 0.001 | 0.05 ± 0.002 | 1.0 ± 0.035 |
| E3 | 4400 ± 123.7 | 1100 ± 3.9 | 2.8 ± 0.10 | 0.47 ± 0.02 | 14 ± 0.5 | 7.5 ± 0.3 | 0.32 ± 0.01 | 0.75 ± 0.005 | 0.065 ± 0.002 | 0.036 ± 0.001 | 0.05 ± 0.002 | 1.0 ± 0.035 |
| E4 | 3000 ± 106.1 | 590 ± 2.1 | 2.5 ± 0.09 | 0.34 ± 0.01 | 10 ± 0.4 | 11 ± 0.4 | 0.26 ± 0.01 | 0.75 ± 0.018 | 0.065 ± 0.002 | 0.023 ± 0.001 | 0.05 ± 0.003 | 2.2 ± 0.078 |
| E5 | 4600 ± 62.2 | 550 ± 1.9 | 2.3 ± 0.08 | 0.39 ± 0.01 | 14 ± 0.5 | 6.8 ± 0.2 | 0.24 ± 0.01 | 0.75 ± 0.023 | 0.05 ± 0.002 | 0.03 ± 0.001 | 0.05 ± 0.002 | 0.76 ± 0.027 |
| E6 | 2100 ± 35.4 | 700 ± 2.5 | 0.15 | 0.94 ± 0.03 | 7.9 ± 0.3 | 5.6 ± 0.2 | 0.79 ± 0.03 | 0.75 ± 0.008 | 0.075 ± 0.003 | 0.02 ± 0.001 | 0.05 ± 0.002 | 1.4 ± 0.049 |
| E7 | 3700 ± 7.1 | 1300 ± 4.6 | 4.6 ± 0.16 | 0.51 ± 0.02 | 17 ± 0.6 | 6.1 ± 0.2 | 0.63 ± 0.02 | 0.75 ± 0.027 | 0.05 ± 0.002 | 0.03 ± 0.001 | 0.05 ± 0.002 | 1.8 ± 0.064 |
| E8 | 2100 ± 35.4 | 510 ± 1.8 | 1.9 ± 0.07 | 0.28 ± 0.01 | 8.1 ± 0.3 | 6.2 ± 0.2 | 0.52 ± 0.02 | 0.75 ± 0.013 | 0.067 ± 0.002 | 0.039 ± 0.001 | 0.05 ± 0.002 | 0.93 ± 0.033 |
| E9 | 3000 ± 14.1 | 440 ± 1.6 | 1.9 ± 0.07 | 0.63 ± 0.02 | 22 ± 0.8 | 7.6 ± 0.3 | 0.25 ± 0.01 | 0.75 ± 0.027 | 0.05 ± 0.002 | 0.044 ± 0.002 | 0.05 ± 0.002 | 0.68 ± 0.024 |
| E10 | 3900 ± 17.7 | 340 ± 1.2 | 1 ± 0.04 | 0.67 ± 0.02 | 0.67 ± 0.0 | 5.8 ± 0.2 | 0.16 ± 0.01 | 0.75 ± 0.018 | 0.051 ± 0.002 | 0.028 ± 0.001 | 0.05 ± 0.002 | 0.55 ± 0.019 |
| (B) Element Concentration (mg/kg) in Myrtus communis L. Leaves | ||||||||||||
| Samples | Mg ± Std | Al ± Std | Cr ± Std | Co ± Std | Ni ± Std | Cu ± Std | As ± Std | Se ± Std | Cd ± Std | Hg ± Std | Tl ± Std | Pb ± Std |
| N1 | 3300 ± 133.6 | 370 ± 1.3 | 1.2 ± 0.04 | 1.5 ± 0.05 | 3.6 ± 0.1 | 6.3 ± 0.2 | 0.24 ± 0.01 | 0.75 ± 0.013 | 0.21 ± 0.007 | 0.049 ± 0.002 | 0.05 ± 0.002 | 0.93 ± 0.033 |
| N2 | 2700 ± 14.1 | 580 ± 2.1 | 1.6 ± 0.06 | 0.44 ± 0.02 | 3.1 ± 0.1 | 5.8 ± 0.2 | 0.19 ± 0.01 | 0.75 ± 0.011 | 0.17 ± 0.006 | 0.02 ± 0.001 | 0.05 ± 0.002 | 1.2 ± 0.042 |
| N3 | 4400 ± 17.7 | 960 ± 3.4 | 3.8 ± 0.13 | 1.9 ± 0.07 | 6.5 ± 0.2 | 5.8 ± 0.2 | 0.42 ± 0.01 | 0.75 ± 0.011 | 0.11 ± 0.004 | 0.02 ± 0.001 | 0.05 ± 0.002 | 1.5 ± 0.053 |
| N4 | 2000 ± 3.5 | 730 ± 2.6 | 3.1 ± 0.11 | 0.79 ± 0.03 | 5 ± 0.2 | 9.1 ± 0.3 | 0.33 ± 0.01 | 0.75 ± 0.012 | 0.15 ± 0.005 | 0.02 ± 0.001 | 0.05 ± 0.002 | 3.9 ± 0.138 |
| N5 | 6000 ± 21.2 | 810 ± 2.9 | 3.9 ± 0.14 | 0.83 ± 0.03 | 5.3 ± 0.2 | 6.3 ± 0.2 | 0.45 ± 0.02 | 0.75 ± 0.014 | 0.13 ± 0.005 | 0.02 ± 0.001 | 0.05 ± 0.002 | 1.4 ± 0.049 |
| N6 | 3500 ± 84.9 | 490 ± 1.7 | 2.9 ± 0.10 | 0.7 ± 0.02 | 24 ± 0.8 | 5.2 ± 0.2 | 0.5 ± 0.02 | 0.75 ± 0.027 | 0.076 ± 0.003 | 0.042 ± 0.001 | 0.05 ± 0.002 | 1.1 ± 0.039 |
| N7 | 3500 ± 38.9 | 700 ± 2.5 | 2.7 ± 0.10 | 1.4 ± 0.05 | 6.9 ± 0.2 | 8.4 ± 0.3 | 0.46 ± 0.02 | 0.75 ± 0.014 | 0.087 ± 0.003 | 0.033 ± 0.001 | 0.05 ± 0.002 | 1.2 ± 0.042 |
| N8 | 2500 ± 8.5 | 240 ± 0.8 | 0.85 ± 0.03 | 0.47 ± 0.02 | 2.6 ± 0.1 | 5.3 ± 0.2 | 0.19 ± 0.01 | 0.75 ± 0.029 | 0.11 ± 0.004 | 0.02 ± 0.001 | 0.05 ± 0.002 | 0.88 ± 0.031 |
| N9 | 2700 ± 7.1 | 470 ± 1.7 | 1.9 ± 0.07 | 1.7 ± 0.06 | 5.6 ± 0.2 | 7 ± 0.2 | 0.27 ± 0.01 | 0.75 ± 0.027 | 0.061 ± 0.002 | 0.02 ± 0.001 | 0.05 ± 0.002 | 1.1 ± 0.039 |
| N10 | 4800 ± 56.6 | 330 ± 1.2 | 1.2 ± 0.04 | 0.21 ± 0.01 | 9.8 ± 0.3 | 6.2 ± 0.2 | 0.22 ± 0.01 | 0.75 ± 0.019 | 0.067 ± 0.002 | 0.036 ± 0.001 | 0.05 ± 0.002 | 0.38 ± 0.013 |
| (C) Element Concentration (mg/kg) in Soil Samples | ||||||||||||
| Samples | Mg ± Std | Al ± Std | Cr ± Std | Co ± Std | Ni ± Std | Cu ± Std | As ± Std | Se ± Std | Cd ± Std | Hg ± Std | Tl ± Std | Pb ± Std |
| S1 | 16,000 ± 141.4 | 28,000 ± 99.0 | 120 ± 4.24 | 15 ± 0.53 | 100 ± 3.5 | 23 ± 0.8 | 6.3 ± 0.22 | 4.1 ± 0.146 | 0.23 ± 0.008 | 0.02 ± 0.001 | 0.15 ± 0.005 | 25 ± 0.884 |
| S2 | 9900 ± 35.4 | 17,000 ± 60.1 | 150 ± 5.30 | 9.6 ± 0.34 | 65 ± 2.3 | 210 ± 7.4 | 4.8 ± 0.17 | 2.9 ± 0.102 | 0.52 ± 0.018 | 0.02 ± 0.001 | 0.095 ± 0.003 | 140 ± 4.950 |
| S3 | 19,000 ± 134.4 | 29,000 ± 102.5 | 120 ± 4.24 | 16 ± 0.57 | 120 ± 4.2 | 25 ± 0.9 | 6.8 ± 0.24 | 5.1 ± 0.180 | 0.28 ± 0.010 | 0.02 ± 0.001 | 0.17 ± 0.006 | 27 ± 0.955 |
| S4 | 12,000 ± 84.9 | 18,000 ± 63.6 | 89 ± 3.15 | 11 ± 0.39 | 70 ± 2.5 | 55 ± 1.9 | 5.4 ± 0.19 | 3.4 ± 0.121 | 0.58 ± 0.021 | 0.035 ± 0.001 | 0.092 ± 0.003 | 67 ± 2.369 |
| S5 | 11,000 ± 77.8 | 18,000 ± 63.6 | 120 ± 4.24 | 9.9 ± 0.35 | 63 ± 2.2 | 24 ± 0.8 | 5 ± 0.18 | 3.5 ± 0.123 | 0.17 ± 0.006 | 0.02 ± 0.001 | 0.086 ± 0.003 | 30 ± 1.061 |
| S6 | 17,000 ± 120.2 | 19,000 ± 67.2 | 140 ± 4.95 | 12 ± 0.42 | 98 ± 3.5 | 17 ± 0.6 | 5.2 ± 0.18 | 3.4 ± 0.122 | 0.18 ± 0.006 | 0.02 ± 0.001 | 0.087 ± 0.003 | 9.1 ± 0.322 |
| S7 | 13,000 ± 91.9 | 24,000 ± 84.9 | 150 ± 5.30 | 13 ± 0.46 | 94 ± 3.3 | 26 ± 0.9 | 6.3 ± 0.22 | 3.6 ± 0.127 | 0.29 ± 0.010 | 0.02 ± 0.001 | 0.12 ± 0.004 | 34 ± 1.202 |
| S8 | 16,000 ± 113.1 | 21,000 ± 74.2 | 170 ± 6.01 | 12 ± 0.42 | 76 ± 2.7 | 24 ± 0.8 | 5.3 ± 0.19 | 3.7 ± 0.130 | 0.25 ± 0.009 | 0.029 ± 0.001 | 0.11 ± 0.004 | 33 ± 1.167 |
| S9 | 19,000 ± 134.4 | 27,000 ± 95.5 | 140 ± 4.95 | 17 ± 0.60 | 130 ± 4.6 | 27 ± 1.0 | 6.7 ± 0.24 | 4.7 ± 0.167 | 0.24 ± 0.008 | 0.02 ± 0.001 | 0.14 ± 0.005 | 9.1 ± 0.322 |
| S10 | 19,000 ± 67.2 | 25,000 ± 88.4 | 150 ± 5.30 | 14 ± 0.49 | 110 ± 3.9 | 20 ± 0.7 | 6.4 ± 0.23 | 4.1 ± 0.145 | 0.19 ± 0.007 | 0.02 ± 0.001 | 0.12 ± 0.004 | 11 ± 0.389 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurshid, C.A.; Mahdi, K.; Ahmed, O.I.; Osman, R.; Rahman, M.; Ritsema, C. Assessment of Potentially Toxic Elements in the Urban Soil and Plants of Kirkuk City in Iraq. Sustainability 2022, 14, 5655. https://doi.org/10.3390/su14095655
Khurshid CA, Mahdi K, Ahmed OI, Osman R, Rahman M, Ritsema C. Assessment of Potentially Toxic Elements in the Urban Soil and Plants of Kirkuk City in Iraq. Sustainability. 2022; 14(9):5655. https://doi.org/10.3390/su14095655
Chicago/Turabian StyleKhurshid, Chrow Ahmed, Karrar Mahdi, Osamah Ibrahim Ahmed, Rima Osman, Mostafizur Rahman, and Coen Ritsema. 2022. "Assessment of Potentially Toxic Elements in the Urban Soil and Plants of Kirkuk City in Iraq" Sustainability 14, no. 9: 5655. https://doi.org/10.3390/su14095655
APA StyleKhurshid, C. A., Mahdi, K., Ahmed, O. I., Osman, R., Rahman, M., & Ritsema, C. (2022). Assessment of Potentially Toxic Elements in the Urban Soil and Plants of Kirkuk City in Iraq. Sustainability, 14(9), 5655. https://doi.org/10.3390/su14095655









